Send us your feedback
Here you can send us feedback on the Maxess-website. Please describe the problem or what’s missing in a clear way, and on what page you found the issue. Thank you so much for your help!
Catalyst under synchrotron light
For most chemical reactions on an industrial scale, catalysts are used to increase the reaction rate, give selectivity regarding the reaction products or lower the reaction temperature. Studie on catalysts by synchrotron radiation has made it possible to study their structure as a whole, which helps companies improve their catalyst.
Hydrogen production by water-gas shift reaction
In this exemplary case study, a catalyst for the water-gas shift reaction was examined. During the water-shift reaction, carbon monoxide (CO) and water (H2O) react to form carbon dioxide (CO2) and hydrogen (H2): CO + H2O ⇌ CO2 + H2. This reaction is widely used in the chemical industry to produce hydrogen, which is then involved in the production of other important substances like methanol or ammonia.
According to the International Energy Agency, the global demand for H2 was over 70 megatons in 2018. The water-shift reaction is quite sensitive to the reaction temperature. The main component of the catalyst examined is magnetite (Fe3O4), an iron oxide mineral accompanied by smaller amounts of chromium for stabilization and copper to further enhance the reaction. The interaction of the components with the reactants and their local structure are key properties, which scientist would like to examine in order to improve the performance of the catalyst.
Identifying structure and oxidation states inside the catalyst
Haldor Topsøe is a Danish multinational chemical company specializing in the development and production of catalysts for the chemical, fertilizer and petroleum industries. For their research on new catalysts they frequently come to large-scale facilities like PETRA III at DESY, where they use the brilliant synchrotron radiation to gain a better understanding of the structure of catalysts and the interaction between their components.
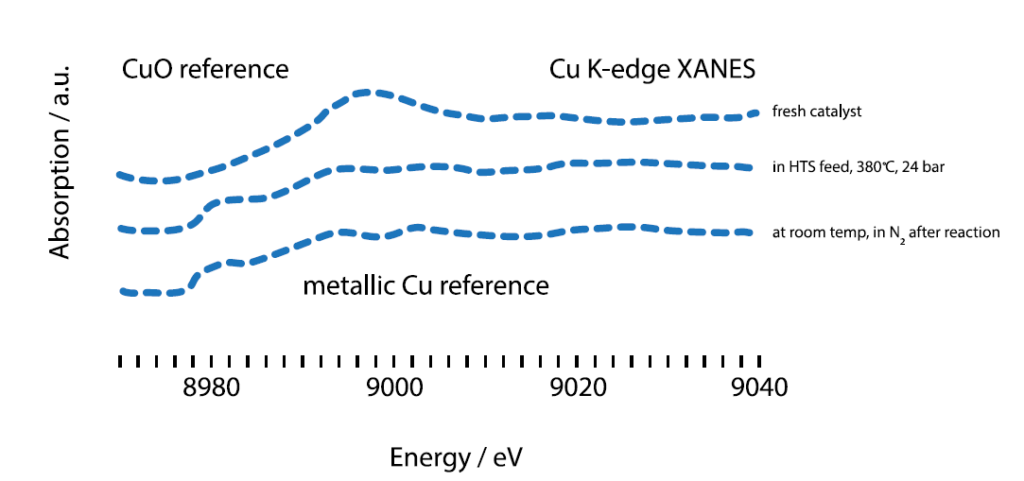
X-ray absorption spectra of the catalyst under three different
conditions: Before the reaction, during the reaction, after post-reaction
washing.
The synchrotron radiation from PETRA III was used for X-ray absorption fine structure (XAFS) experiments to examine the catalyst under different conditions. It helped to resolve the different energy states of copper during the catalysis, revealing important interactions between the catalyst’s components. The good resolution of XAFS made it possible to distinguish between different oxidation levels of certain elements. Aside from XAFS’s ability to resolve elements in near energetic states, XAFS also provides information about the local structure and geometry of the samples of interest. The knowledge gained during the experiments ranged from oxidation states, to geometry and local environment, allowing conclusions to be drawn about the active species, the role of Cu and the movement of Cr ions during the reaction. With their new understanding, Haldor Topsøe is now ready to develop a better catalyst for one of most important reactions in industrial chemistry.
Case Details
Haldor Topsøe



