Send us your feedback
Here you can send us feedback on the Maxess-website. Please describe the problem or what’s missing in a clear way, and on what page you found the issue. Thank you so much for your help!
New cancer drugs developed with the help of X-ray crystallography
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one out of six deaths yearly. In a research project carried out in 2019-2020, scientists from the drug discovery company Sprint Bioscience and researchers from MAX IV used X-ray crystallography to take important steps toward developing new and more efficient cancer drugs.
Cancers can affect any part of the body. The most common forms are breast, lung and colon cancer. Cancer begins when one or more genes in a cell mutate. The mutation creates an abnormal protein or can prevent a protein’s formation. The cells grow beyond their usual boundaries and can also invade other parts of the body, a process called metastasis.
Identifying fragments that can bind cancer protein
The first step in a fragment-based drug discovery process is identifying small molecules (fragments) that bind the targeted cancer protein. In this step, the protein is screened using various biophysical methods against a number of fragments in order to develop suitable drug candidates.
Sprint Bioscience has designed and formulated a fragment library of 300 chemicals suitable for X-ray-based Fragment Screening. As part of the collaboration, the researchers in the FragMAX team at MAX IV optimised and developed a protein crystallisation system corresponding to the cancer protein chosen by Sprint Bioscience. The crystallised protein was then screened against the library of 300 fragments.
Today, X-ray crystallography is one of the most powerful methods to perform this type of screening. It enables researchers to test many crystals and provides detailed data.
Novel knowledge gained using X-ray crystallography
The screening was performed at a very high fragment concentration, 90 mM, making it possible to detect not previously identified low-affinity protein binders. The result directly provides a three-dimensional model of the interaction between the fragments and the protein, which is essential to developing the fragments into drug candidates.
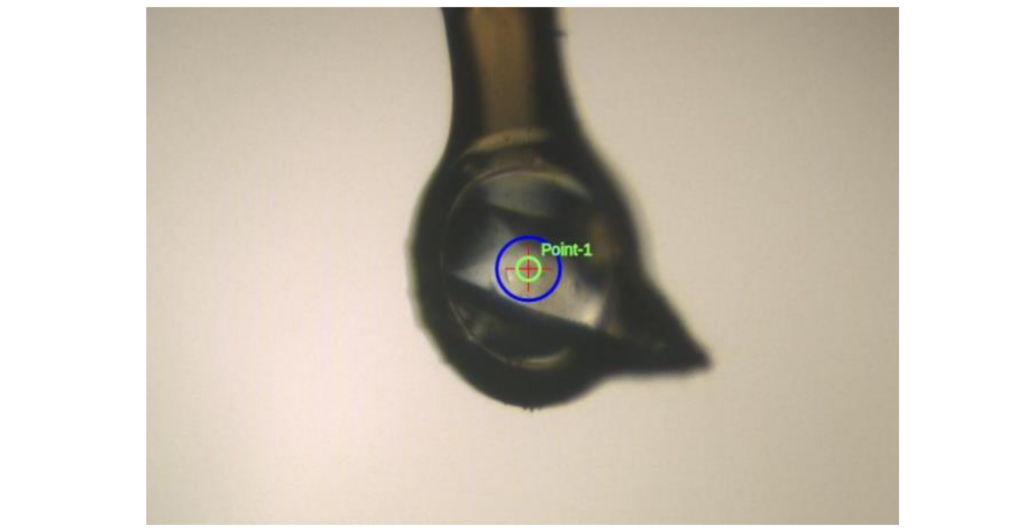
The results showed that, in total, 27 fragments bound to the cancer protein in different ways. They were identified by analysing 272 data sets collected over one week. Among these, eleven new fragment interactions were detected for the first time.
All data was collected at the BioMAX beamline at MAX IV and processed using the FragMAX App.
Towards new knowledge and more efficient drugs
The collaboration has yielded new knowledge to bring drug development one step further toward new cancer drugs. The experiment has also confirmed that X-ray crystallography could pave the way for developing better, more efficient drugs, allowing companies to find starting points for rational drug development quickly and cost-effectively.
“The collaboration with Sprint Bioscience has been crucial for optimising the FragMAX platform to the requirements of drug development companies. The process is now very much streamlined and can be readily applied to many other, medically important targets.”
Tobias Krojer, FragMAX project manager and beamline scientist, MAX IV
Contact Partners
Case Details
Sprint Bioscience
BioMAX




